
Vaccination and dangers ahead
Official data from the On Data in World (in graphic) website 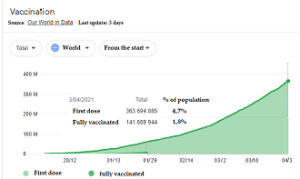 indicate a total vaccination in Brazil of 10.2 million people up to 3 days ago, while the world average is at the level of 6.5% and in countries like France, Italy and Germany they are inexplicable. rate close to 11% (even the WHO complained).
indicate a total vaccination in Brazil of 10.2 million people up to 3 days ago, while the world average is at the level of 6.5% and in countries like France, Italy and Germany they are inexplicable. rate close to 11% (even the WHO complained).
Only Israel close to 60% and the United Kingdom close to 50% have acceptable rates, in Latin America only Chile reached 30% and Uruguay to 20%, but it should be noted that Chile has a much smaller population of 18 million (less than the city of São Paulo), and Uruguay 3.5 million (slightly larger than Belo Horizonte if counting the cities Betim, Contagem that are neighboring), Brazil is close to 10% counting the first dose according to data from the same site (on Data in World ).
Two dangers strongly threaten the contamination rates: the new strain that spreads more quickly and the cold that should arrive soon, according to experts from Butantã in Sao Paulo, it is not possible to speed up the vaccine production process, but on the other hand, FioCruz in Rio de Janeiro promises to deliver 1 million daily doses, would arrive at the end of April with at least 40 million vaccinated with the first dose (this would be close to 20% of the population), and hope that the cold will delay, not counting the shipments that will come from the abroad and Butantã itself.
Fortunately the weather has been hot, despite cold fronts threatening in the south, the expectation for the next 15 days (until April 20, therefore), is to stay warm. although it is rainy, but there is no correct weather data, the forecasts are not always right.
It is hoped that a vaccination will be able to stop the successive increase of infections, we have broken several records of infection and deaths, and the aggressiveness of the new strain acts in these two aspects.
An innovative drug is announced on the market, and filed the order on Anvisa on March 30, it is a combination of monoclonal antibodies bamlanivimab and etesevimab that was developed by the pharmaceutical company Eli Lilly, and can help in mild cases and people who have respiratory problems , is the first drug really valid in prevention and in mild cases that have respiratory comorbidities, it can reach the market in 30 days.
This medicine has a powerful asset, since it has already been released by the Food and Drug Administration (FDA), the drug regulator in the USA, in short it is necessary to think of creative solutions and preventive measures cannot be ruled out, of course they are really valid.









